-
Study
-
Quick Links
- Open Days & Events
- Real-World Learning
- Unlock Your Potential
- Tuition Fees, Funding & Scholarships
- Real World Learning
-
Undergraduate
- Application Guides
- UCAS Exhibitions
- Extended Degrees
- School & College Outreach
- Information for Parents
-
Postgraduate
- Application Guide
- Postgraduate Research Degrees
- Flexible Learning
- Change Direction
- Register your Interest
-
Student Life
- Students' Union
- The Hub - Student Blog
- Accommodation
- Northumbria Sport
- Support for Students
-
Learning Experience
- Real-World Learning
- Research-enriched learning
- Graduate Futures
- The Business Clinic
- Study Abroad
-
-
International
International
Northumbria’s global footprint touches every continent across the world, through our global partnerships across 17 institutions in 10 countries, to our 277,000 strong alumni community and 150 recruitment partners – we prepare our students for the challenges of tomorrow. Discover more about how to join Northumbria’s global family or our partnerships.
View our Global Footprint-
Quick Links
- Course Search
- Undergraduate Study
- Postgraduate Study
- Information for Parents
- London Campus
- Northumbria Pathway
- Cost of Living
- Sign up for Information
-
International Students
- Information for International Students
- Northumbria and your Country
- International Events
- Application Guide
- Entry Requirements and Education Country Agents
- Global Offices and Regional Teams
- English Requirements
- English Language Centre
- International student support
- Cost of Living
-
International Fees and Funding
- International Undergraduate Fees
- International Undergraduate Funding
- International Masters Fees
- International Masters Funding
- International Postgraduate Research Fees
- International Postgraduate Research Funding
- Useful Financial Information
-
International Partners
- Agent and Representatives Network
- Global Partnerships
- Global Community
-
International Mobility
- Study Abroad
- Information for Incoming Exchange Students
-
-
Business
Business
The world is changing faster than ever before. The future is there to be won by organisations who find ways to turn today's possibilities into tomorrows competitive edge. In a connected world, collaboration can be the key to success.
More on our Business Services-
Business Quick Links
- Contact Us
- Business Events
- Research and Consultancy
- Education and Training
- Workforce Development Courses
- Join our mailing list
-
Education and Training
- Higher and Degree Apprenticeships
- Continuing Professional Development
- Apprenticeship Fees & Funding
- Apprenticeship FAQs
- How to Develop an Apprentice
- Apprenticeship Vacancies
- Enquire Now
-
Research and Consultancy
- Space
- Energy
- AI and Tech
- CHASE: Centre for Health and Social Equity
- NESST
-
-
Research
Research
Northumbria is a research-rich, business-focused, professional university with a global reputation for academic quality. We conduct ground-breaking research that is responsive to the science & technology, health & well being, economic and social and arts & cultural needs for the communities
Discover more about our Research-
Quick Links
- Research Peaks of Excellence
- Academic Departments
- Research Staff
- Postgraduate Research Studentships
- Research Events
-
Research at Northumbria
- Interdisciplinary Research Themes
- Research Impact
- REF
- Partners and Collaborators
-
Support for Researchers
- Research and Innovation Services Staff
- Researcher Development and Training
- Ethics, Integrity, and Trusted Research
- University Library
- Vice Chancellors Fellows
-
Research Degrees
- Postgraduate Research Overview
- Doctoral Training Partnerships and Centres
- Academic Departments
-
Research Culture
- Research Culture
- Research Culture Action Plan
- Concordats and Commitments
-
-
About Us
-
About Northumbria
- Our Strategy
- Our Staff
- Our Schools
- Place and Partnerships
- Leadership & Governance
- University Services
- Northumbria History
- Contact us
- Online Shop
-
-
Alumni
Alumni
Northumbria University is renowned for the calibre of its business-ready graduates. Our alumni network has over 253,000 graduates based in 178 countries worldwide in a range of sectors, our alumni are making a real impact on the world.
Our Alumni - Work For Us
 MARIE CURIE ACTIONS
MARIE CURIE ACTIONS
Project H2020-MSCA-IF-2015 – GA - 705944
High Performance Seasonal Solar Energy Latent Heat Thermal Storage Using Low Grade, Low Melting Temperature Metallic (THERMOSTALL)
Project duration: 24 months (2016-2018); Grant value: € 195k
Funded by EUROPEAN COMMISSION Research Executive Agency
PI: Prof. K Mahkamov; MC Research Fellow: Dr. Carolina Costa;
Aim and Objectives
Energy storage technologies have long been a subject of great interest to both academia and industry. The aim of this project is to develop a novel, cost effective and high performance Latent Heat Thermal Energy Storage System (LHTESS) for seasonal accumulation of solar energy in increased quantities.
The major barrier for currently used organic, salt and salt hydrates based Phase Change Materials is their very low thermal conductivity coefficient and density, which results in the relatively large volumes of storage systems and difficulties in achieving the necessary rates of thermal energy re-charge and discharge, even when using advanced heat exchangers.
The new approach to overcome the above issues is the deployment of low grade, low melting temperature metallic alloys (ELMTAs). The ELMTAs are currently produced for application in other industrial sectors (e.g. electronics) and have not been actively considered for the thermal energy accumulation with the exception of very limited studies. Their heat conduction is two orders of magnitude greater than that of conventional PCMs and they are stable and can provide the thermal storage capacity which is 2-3 times greater per unit of volume due to the greater densities.
The project consists of both theoretical and experimental investigations. A range of low grade ELMTAs for application in LHTESS have been selected and their thermophysical properties are being studied using Differential Scanning Calorimetry and thermal conductivity analysis. Thermal cycling tests of such alloys are also being conducted.
Numerical investigations of heat transfer and flow in the LHTESS with ELMTAs will be performed. Experimental studies of heat transfer and flow in a laboratory prototype of the LHTESS with ELMTAs will be conducted. As outcomes of investigations, dimensionless heat transfer correlations will be derived and design recommendations for a practical solar energy seasonal LHTESS with the low grade ELMTA will be produced for a project industrial partner.
Brief description of Literature analysis
Through literature analysis shows that here is no a clear consensus about the use of metallic materials as PCMs (MPCMs) for LHTES. The low thermal conductivity of the common PCMs, leads to the need for bigger heat exchange surfaces or embedding metal or graphite/carbon structures into the PCMs to enhance their performance. Such the approach negatively affects the feasibility of LHTES [1, 2]. On the other hand, metallic PCMs (MPCMs) have a significant advantage in thermal conductivity and this parameter does not require enhancing [3-8]. However, there are a number of research works [9-13] which highlight the low heat of fusion per unit weight [4, 6-8, 12, 14], high degree of sub-cooling and their relatively high cost, compared to that of more conventional PCMs. To date, metal alloys have not been endorsed as effective PCMs although they have also some additional advantages such as high density, low corrosiveness and small volume change during melting and solidification [17].
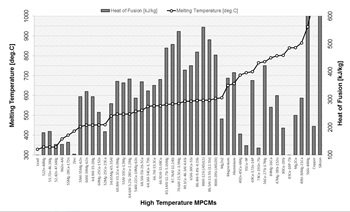
For high temperature applications aluminium alloys were investigated in [15] as potential PCMs (>300 °C) because of their high latent heat and good thermal stability.
Information on the lowest reported values of the heat of fusion for high temperature MPCMs is summarised in Fig. 1. It can be seen that eutectic aluminium alloys have the highest heat of fusion in the group considered. With regard to the cost and availability of the materials, prices for Mg, Zn, Si and Al are approximately between 2 and 3 $/kg and these materials are widely used in various industrial applications [16].
For the mid- temperature range (between 48 and 124 °C), metals with the low melting temperature or alloys based on elements such as Bi, Pb, Sn, Cd, In, Ga, Zn, Sb etc., are available. Low-melting-point alloys are extensively used in the fields of materials processing, electronics, electrical automatic control, continuous casting simulation, welding etc. [17, 18]. Bismuth is one of the major components of many fusible alloys, which strongly influence the value of melting point, and has the unique characteristic, namely it expands during solidification process [19]. Summary of information on melting temperatures and heat of fusion of some mid-temperature MPCMs, investigated in a number of studies, is presented in Fig. 2.
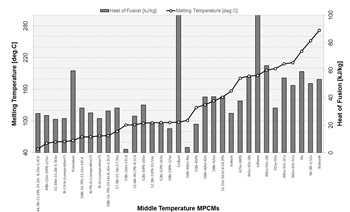
Many of alloys, which could be deployed in the mid-temperature range, contain harmful elements, such as lead (Pb). Lead is considered as one of the top 17 chemicals, harmful for human life and environment. This is highlighted by the Environmental Protection Agency (EPA) and the European Union's RoHS (Restriction of Hazardous Substances in Electrical and Electronic Equipment). Therefore, lead containing materials should be treated with special protection measures put in place.
Lead-free solders were studied in [20-31] with relevant databases have been created [32-34]. Three most frequently referenced lead-free alloys to substitute the Pb-Sn eutectic composition solder are Sn–Ag–Cu (near eutectic), Sn–Zn (eutectic) and Sn–Bi (eutectic) composites. At present, the Sn– (3–4) wt% Ag–(0.5–0.9) wt% Cu alloy is preferred for application in soldering [21]. This is due to a number of factors, that affect the soldering process, namely mechanical properties, melting point, cost, availability and wetting property. Furthermore, Pb-free solders are used because of their cost competitiveness. in order to maintain acceptable costs of production in electronic industry. On elemental basis, Pb and Zn are the cheapest metals, whilst In is more expensive than Ag.
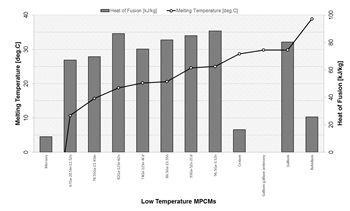 At the low-temperature
range (about 40 °C), liquid metals or low melting point alloys have been
studied and used for diverse applications, mainly as thermal management in
electronic devices. Room temperature liquid metals seem to meet most of the
requirements for meeting thermal comfort requirements [6]. Fig. 3 presents summary of gathered information on the melting temperatures and heat of
fusion for such the low-temperature MPCMs.
At the low-temperature
range (about 40 °C), liquid metals or low melting point alloys have been
studied and used for diverse applications, mainly as thermal management in
electronic devices. Room temperature liquid metals seem to meet most of the
requirements for meeting thermal comfort requirements [6]. Fig. 3 presents summary of gathered information on the melting temperatures and heat of
fusion for such the low-temperature MPCMs.
Selected ELMTAs and their Characterization
Eight commercially available ELMTAs were selected as potential candidates as PCMs for the seasonal thermal storage (see Fig. 4):
|
Name |
Chemical composition |
Melting Point |
|
Alloy 158F |
50Bi-26.7Pb-13.3Sn-10Cd |
70 |
|
Alloy 174F |
57Bi-26In-17Sn (lead-free) |
79 |
|
Alloy 203F |
52.5Bi- 32Pb-15.5Sn |
95 |
|
Alloy 281F |
58.0Bi- 42.0Sn (lead-free) |
138 |
|
Alloy 62S |
62.5Sn- 36.1Pb- 1.4Ag |
179 |
|
Alloy 63/37 |
63.0Sn- 37.0Pb |
183 |
|
Alloy 96S |
96.5Sn- 3.5Ag (lead-free) |
221 |
|
Alloy 99C |
99.3Sn- 0.7Cu |
227 |
 Alloys listed above are widely
used commercially in soldering processes and are described in the standard specifications for Low Melting
Point Alloys ASTM B774 [35] (<183 °C) and in Soft solder alloys BS-EN-ISO 9453 (<450 °C) [36]. The impurity
level in these commercial alloys is <0.15
wt.%.
Alloys listed above are widely
used commercially in soldering processes and are described in the standard specifications for Low Melting
Point Alloys ASTM B774 [35] (<183 °C) and in Soft solder alloys BS-EN-ISO 9453 (<450 °C) [36]. The impurity
level in these commercial alloys is <0.15
wt.%.
Differential Scanning Calorimetry analysis was used to determine the latent heat, phase change temperature and heat capacity of the selected ELMTAs as a function of the temperature. DSC measurements were performed in argon gas environment using a Setaram EVO131 Differential Scanning Calorimeter. The DSC calibration was performed following the Standard Test Method ASTM B1269 [37]. During the measurements the DSC apparatus was purged with argon, all the weigh measurements were carried out with the accuracy of ± 0.01 mg. The calorimeter was calibrated with the melting point and enthalpy of fusion for high-purity Sn. The ELMATs specimens were placed in aluminium crucibles (30 mm) and samples were thermally cycled 2-3 times with the heating and cooling rates of 2 K/min. The maximum heating temperatures were set about 50 K higher than the melting temperatures.
The above data obtained for ELMTAs from literature review and experimental characterisation of thermal properties was saved as database.
Figures 5 and 6 show samples of results from DSC measurements for one of the selected ELMATs, namely Alloy 158F. Bolton 158F and Roto 158F are the same alloy, supplied by two different companies. Heating rate used in measurements was 5 K/min.
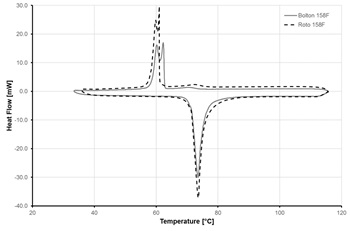
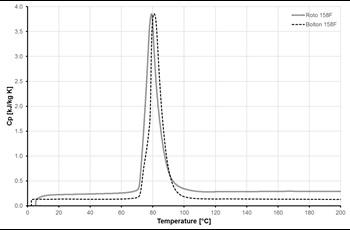
Fig. 5 demonstrates the significant temperature difference in the melting and crystallisation processes (75 and 60 °C, respectively) which is caused by the slow rate of crystal structure formation in metallic alloys.
The variation of the specific heat capacity of the Alloy 158F as function of temperature, is shown in Fig.6 (the heating rate used was 20 K/min). This diagram indicates that the phase change process takes place in the temperature range between 70 and 90 °C.
Most of MPCMs may have this considerable temperature difference between melting and solidification temperatures, which should be taken into account during thermal storage system design and exploitation phases.
Overall metallic alloys provide much superior performance in terms of heat charging and discharging times. Figs. 7 and 8 below show comparison of this performance during discharging process (solidification) for Tin and Solar Salt based thermal storages with the same capacity. It can be clearly seen that during the same period of time significantly more heat was delivered to the user when the storage was metallic alloy based.
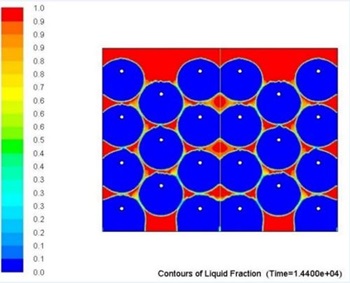
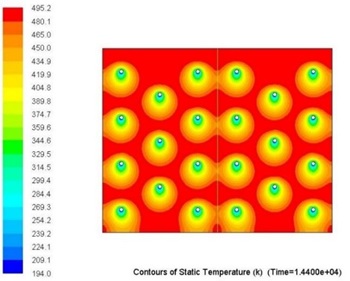
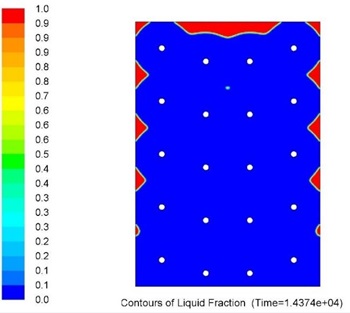
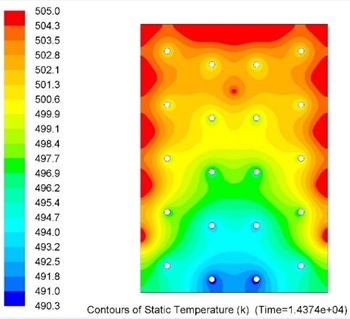
Current work
Several alloys were selected for further investigations to determine the influence of higher levels of impurity (>1wt.%) on their thermophysical properties as MPCMs.
Presently, measurements of the thermal conductivity as a function of temperature for the selected MPCMS is ongoing.
The short-term thermal stability experiments (100 cycles) using the DSC technique are being run.
Numerical and experimental investigations of heat transfer and flow in the laboratory prototype of LHTESS with ELMTAs for deriving heat transfer correlations.
Practical Applications
Results obtained will be used in the development of a novel effective Solar Thermal Energy Storage systems for a small solar power plant Innova MicroSolar - (Project 723596 - H2020-EE-2016-2017/H2020-EE-2016-RIA-IA, 2016-2020).
Additionally, outcomes will be used in R&D activities of the industrial partner in THERMOSTALL Project.
References
[1] F. Agyenim, N. Hewitt, P. Eames, and M. Smyth, "A review of materials, heat transfer and phase change problem formulation for latent heat thermal energy storage systems (LHTESS)," Renewable and Sustainable Energy Reviews, vol. 14, pp. 615-628, 2010.
[2] J. P. Kotzé, "Thermal energy storage in metallic phase change materials," Doctor of Philosophy, Faculty of Engineering, Stellenbosch University, 2014.
[3] M. M. Kenisarin, "High-temperature phase change materials for thermal energy storage," Renewable and Sustainable Energy Reviews, vol. 14, pp. 955-970, 2010.
[4] H. Ge and J. Liu, "Phase change effect of low melting point metal for an automatic cooling of USB flash memory," in Frontiers in Energy vol. 6, ed, 2012, pp. 207-209.
[5] M. Liu, W. Saman, and F. Bruno, "Review on storage materials and thermal performance enhancement techniques for high temperature phase change thermal storage systems," Renewable and Sustainable Energy Reviews, vol. 16, pp. 2118-2132, 2012.
[6] H. Ge, H. Li, S. Mei, and J. Liu, "Low melting point liquid metal as a new class of phase change material: An emerging frontier in energy area," Renewable and Sustainable Energy Reviews, vol. 21, pp. 331-346, 2013.
[7] S. A. Mohamed, F. A. Al-Sulaiman, N. I. Ibrahim, M. H. Zahir, A. Al-Ahmed, R. Saidur, et al., "A review on current status and challenges of inorganic phase change materials for thermal energy storage systems," Renewable and Sustainable Energy Reviews, vol. 70, pp. 1072-1089, 2017.
[8] B. Cárdenas and N. León, "High temperature latent heat thermal energy storage: Phase change materials, design considerations and performance enhancement techniques," Renewable and Sustainable Energy Reviews, vol. 27, pp. 724-737, 2013.
[9] M. M. Farid, A. M. Khudhair, S. A. K. Razack, and S. Al-Hallaj, "A review on phase change energy storage: materials and applications," Energy Conversion and Management, vol. 45, pp. 1597-1615, 2004.
[10] A. M. Khudhair and M. M. Farid, "A review on energy conservation in building applications with thermal storage by latent heat using phase change materials," Energy Conversion and Management, vol. 45, pp. 263-275, 2004.
[11] A. Gil, M. Medrano, I. Martorell, A. Lázaro, P. Dolado, B. Zalba, et al., "State of the art on high temperature thermal energy storage for power generation. Part 1—Concepts, materials and modellization," Renewable and Sustainable Energy Reviews, vol. 14, pp. 31-55, 2010.
[12] A. Sharma, V. V. Tyagi, C. R. Chen, and D. Buddhi, "Review on thermal energy storage with phase change materials and applications," Renewable and Sustainable Energy Reviews, vol. 13, pp. 318-345, 2009.
[13] N. Soares, J. J. Costa, A. R. Gaspar, and P. Santos, "Review of passive PCM latent heat thermal energy storage systems towards buildings’ energy efficiency," Energy and Buildings, vol. 59, pp. 82-103, 2013.
[14] T. Khadiran, M. Z. Hussein, Z. Zainal, and R. Rusli, "Advanced energy storage materials for building applications and their thermal performance characterization: A review," Renewable and Sustainable Energy Reviews, vol. 57, pp. 916-928, 2016.
[15] J. Q. Sun, R. Y. Zhang, Z. P. Liu, and G. H. Lu, "Thermal reliability test of Al–34%Mg–6%Zn alloy as latent heat storage material and corrosion of metal with respect to thermal cycling," Energy Conversion and Management, vol. 48, pp. 619-624, 2007.
[16] P. Blanco-Rodríguez, J. Rodríguez-Aseguinolaza, E. Risueño, and M. Tello, "Thermophysical characterization of Mg–51%Zn eutectic metal alloy: A phase change material for thermal energy storage in direct steam generation applications," Energy, vol. 72, pp. 414-420, 2014.
[17] K. Zhou, Z. Tang, Y. Lu, T. Wang, H. Wang, and T. Li, "Composition, Microstructure, Phase Constitution and Fundamental Physicochemical Properties of Low-Melting-Point Multi-Component Eutectic Alloys," Journal of Materials Science & Technology, vol. 33, pp. 131-154, 2017.
[18] Z. Mei, H. A. Holder, and H. A. V. Plas. (1996) Low-Temperature Solders. Hewlett-Packard Journal.
[19] I. Corporation, "How to Use Fusible Alloys," INDIUM Corporation.
[20] M. Abtew and G. Selvaduray, "Lead-Free Solders in Microelectronics," Master Science and Engineering, vol. 27, pp. 95-141, 2000.
[21] K. Suganuma, "Advances in lead-free electronics soldering," Current Opinion in Solid State and Materials Science, vol. 5, pp. 55–64, 2001.
[22] H. MAVOORI, A. G. RAMIREZ, and S. JIN, "Lead-Free Universal Solders for Optical and Electronic Devices," Journal of ELECTRONIC MATERIALS, vol. 31, 2002.
[23] D. T. Siewert, D. S. Liu, D. D. R. Smith, and M. J. C. Madeni, "Properties of Lead-Free Solders," NIST2002.
[24] F. S. Mhiaoui, "Physical properties of lead free solders in liquid and solid state," doctor rerum naturalium, Technischen Universität Chemnitz, 2007.
[25] C. Handwerker, U. Kattner, M. Gaithersburg, K.-W. Moon, and M. Gaithersburg, "Chapter 2: Fundamental Properties of Pb-Free Solder Alloys," in Lead-Free Soldering, ed, 2007.
[26] A. A. El-Daly, Y. Swilem, M. H. Makled, M. G. El-Shaarawy, and A. M. Abdraboh, "Thermal and mechanical properties of Sn–Zn–Bi lead-free solder alloys," Journal of Alloys and Compounds, vol. 484, pp. 134-142, 2009.
[27] M. Palcut, J.Sopoušek, L. Trnková, M. Turňa, E.Hodúlová, J. Janovec, et al., "Thermal analysis of selected tin-based lead-free solder alloys," Kovove Mater., vol. 47, pp. 43-50, 2009.
[28] Yucai Hu, Fengjing Cao, Fangxiao Li, G. Ni, and a. X. Cui, "RESEARCH PROGRESS ON LEAD-FREE SOLDERS," 150 Rev. Adv. Mater. Sci., vol. 29, pp. 150-155, 2011.
[29] A. A. El-Daly, A. E. Hammad, G. S. Al-Ganainy, and A. A. Ibrahiem, "Design of lead-free candidate alloys for low-temperature soldering applications based on the hypoeutectic Sn–6.5Zn alloy," Materials & Design (1980-2015), vol. 56, pp. 594-603, 2014.
[30] Y. Shu, K. Rajathurai, F. Gao, Q. Cui, and Z. Gu, "Synthesis and thermal properties of low melting temperature tin/indium (Sn/In) lead-free nanosolders and their melting behavior in a vapor flux," Journal of Alloys and Compounds, vol. 626, pp. 391-400, 2015.
[31] A. A. El-Daly, A. M. El-Taher, and S. Gouda, "Development of new multicomponent Sn–Ag–Cu–Bi lead-free solders for low-cost commercial electronic assembly," Journal of Alloys and Compounds, vol. 627, pp. 268-275, 2015.
[32] Z. Moser, W. Gąsior, A. Dębski, and J. Pstruś, SURDAT: DATABASE OF LEAD - FREE SOLDERING MATERIALS. Kraków, 200.
[33] M. C. Schmetterer, P. A. Mikula, and P. H. Ipser, "DATABASE FOR PROPERTIES OF LEAD-FREE SOLDER ALLOYS," 2006.
[34] W. Gąsior, Z. Moser, A. Dębski, and T. Siewert, "Integration of the NIST and SURDAT Databases on Physical Properties of Lead-Free Solder Alloys," International Journal of Thermophysics, vol. 31, pp. 502-512, 2010.
[35] ASTM, "ASTM B 774 – 00: Low Melting Point Alloys (Standard)," ed, 2000.
[36] BS, "BS EN 29453-1994: Soft solder alloys — Chemical compositions and forms (Standard)," ed, 1994.
[37] ASTM, "ASTM E1269: Standard Test Method for Determining Specific Heat Capacity by Differential Scanning Calorimetry," ed.
Contacts
Emails: khamid.mahkamov@northumbria.ac.uk Phone: + 44 191 243 7510
carolina.costa@northumbria.ac.uk Phone: + 44 191 243 7649
Latest News and Features

Northumbria expands results day support for students
Northumbria University is expanding and enhancing the support it provides to students receiving…

Northumbria University to host prestigious international conference on GPR technology
Scientists, engineers and researchers using and developing Ground Penetrating Radar (GPR) technology…

Northumbria STEM outreach project marks major milestone
An outreach group from Northumbria University is celebrating a decade of STEM engagement in…

EXPERT COMMENT: AI is gobbling up water it cannot replace – I’m working on a solution
In this article originally written for The Conversation*, Dr Muhammad Wakil Shahzad, Associate…
Northumbria awarded EU funding for sustainable 3D-printed construction research
Northumbria University has been awarded a prestigious Marie Skłodowska-Curie Actions (MSCA)…

Northumbria wins recognition for expanding access to higher education
Northumbria University has been named Higher Education Institution of the Year at a prestigious…

Global recognition for groundbreaking green battery technology
A biodegradable battery developed by researchers at Northumbria University has won a major…
Upcoming events

Commercialising SHAPE Innovations and Impact
Northumbria University
-

